Laboratory Execution System
Enforce lab compliance with LabVantage LES
Follow procedures, streamline processes, and strengthen quality control
LabVantage understands that you manage a wide variety of complex tests and experiments. You also track many types of data, and a large volume and velocity of data; capture and report results; and often make data available for further research, product development, and manufacturing decisions. Following SOPs and quality control procedures can be tedious – especially in paper-based labs.
Used most often in quality manufacturing environments, LabVantage LES defines and guides flawless conformance to process protocols, managing your lab’s operations. It enforces SOPs throughout the manufacturing testing workflow. Workflow execution templates enforce compliance with quality and regulatory requirements.
LabVantage LES works within the LabVantage platform – fully integrated with our LIMS, Electronic Lab Notebook (ELN), and Scientific Data Management System (SDMS) – to support digital transformation, up-leveling your organization’s output and efficiency. Count on LabVantage LES and ELN to:
- Increase efficiency through paperless workflow, automating steps, and fostering quality control and compliance
- Support cross-functional teams sharing data, eliminating silos, and facilitating collaboration
- Configure security, permissions, data access, and audit trails that are consistent with your LIMS
- Access data from anywhere, at any time, with mobile-enabled browser-based solutions that are fully HTML5-compliant
- Eliminate client-side installation, validation, or maintenance
Questions about use cases for LabVantage LES?
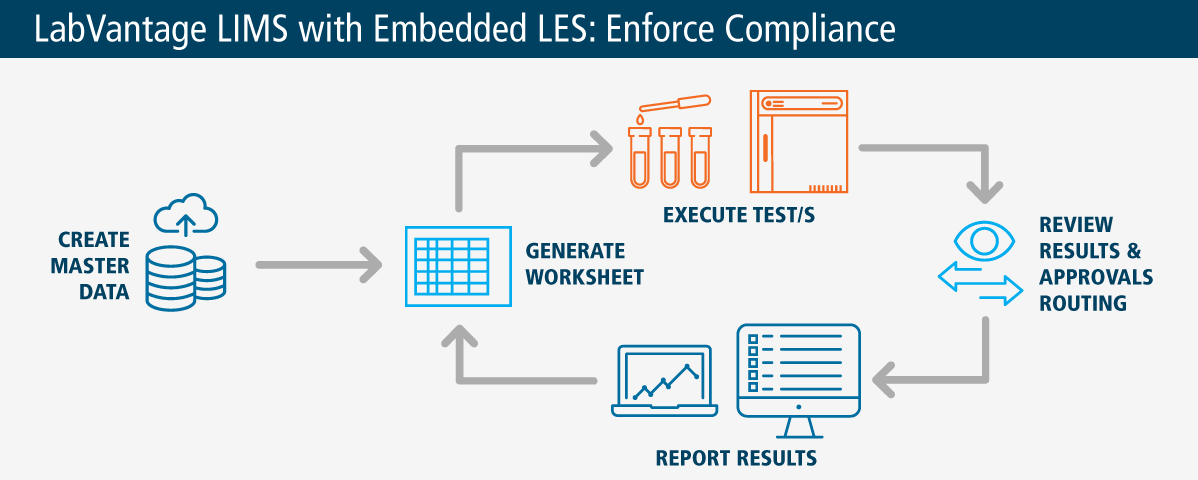
Integrated LES for Peace of Mind
A Lab Execution System, part of your LabVantage platform, reduces data entry and eliminates unnecessary paper records for test execution workflows. Electronic worksheets capture all required data and guide users through the proper execution of lab tests.
Why LabVantage Lab Execution System
LabVantage LES helps product manufacturers ensure they consistently follow standard operational protocols, as well as industry and regulatory requirements so products are made according to exact quality and compliance mandates. The tool prompts users through defined best practices—which can be personalized according to product specifications and company SOPs. The solution guides technicians through workflows with reminders to check for common errors, such as equipment calibration, expired reagents, or insufficient sample size. Specific control factors required by your organization can be incorporated, such as environmental conditions, batch formulas, or any customer-requested variables. Reports needing approvals are automatically sent to managers, saving time.
Key Attributes of LabVantage LES
- Testing methods are easily defined at the time of deployment or as new tests are introduced
- Worksheets and templates allow you to order a test for a single sample or multiple samples at once
- The system guides technicians through steps and protocols, including requirements for calibrated instruments and consumables
- Completed worksheets can be routed for approval through eSignatures, ensuring audit trail compliance
- All activities in a procedure are logged and time-stamped, giving colleagues and managers the ability to review and approve steps as needed
- Reports can be easily generated so you can complete certificates, communicate test results, populate dashboards, or route to business analysts for further study
- Standard templates expedite the documentation process, allowing you to personalize, format, and export to PDF and Word for publishing



